- March 24, 2014
- Taiho Pharmaceutical Co., Ltd.
Taiho's Lonsurf® (trifluridine and tipiracil hydrochloride) Tablets Approved in Japan for Treatment in Advanced Metastatic Colorectal Cancer
Taiho Pharmaceutical Co., Ltd. (HQ: Tokyo, President: Masayuki Kobayashi) announced today that it has obtained approval in Japan from the Ministry of Health, Labour and Welfare to manufacture and market the oral combination anticancer drug ''Lonsurf® combination tablet T15, T20'' (nonproprietary names: trifluridine and tipiracil hydrochloride; development code: TAS-102), for the treatment of patients with unresectable advanced or recurrent colorectal cancer (only if refractory to standard therapies).
Japan is the first country in the world to grant marketing authorization for Lonsurf. The approval is based primarily on the results of a randomized, double blind placebo controlled Phase II clinical trial conducted in Japan (J003-10040030). Taiho is conducting a global Phase III clinical trial (RECOURSE) on patients with metastatic colorectal cancer refractory to standard chemotherapies.
Lonsurf is a combination drug of trifluridine (FTD) and tipiracil hydrochloride (TPI). FTD is an antineoplastic nucleoside analog, which is incorporated directly into DNA, thereby interfering with the function of DNA. The blood concentration of FTD is maintained via TPI, which is an inhibitor of the FTD-degrading enzyme thymidine phosphorylase.
Taiho Pharmaceutical is proud to make Lonsurf available to physicians in Japan as a new treatment option for patients with metastatic colorectal cancer refractory to standard therapies.
Product Summary (for the Japanese market)
Brand name
Lonsurf® combination tablet T15, T20
Nonproprietary name
Trifluridine and tipiracil hydrochloride combination tablet
Indications & Efficacy
Unresectable advanced or recurrent colorectal cancer (only if refractory to standard therapies)
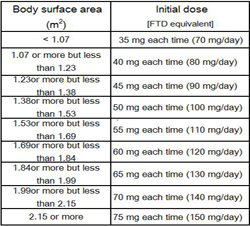
Use & Dosage
Usually, the initial dose (single dose) for adults is defined as the standard dose (approximately 35 mg/m2/dose of FTD) according to body surface area. Lonsurf is administered twice daily, after breakfast and after the evening meal, for five consecutive days, followed by a two-day rest. After repeating the above twice, a 14-day rest follows, completing one course, which is then repeated.The dose can be decreased or increased according to the patient's condition.
Information in this news release was current as of the original release date.
Taiho Pharmaceutical's news releases are intended to provide information to the media. It may contain information about ethical drugs or products under development, however information contained in the news releases are not intended to constitute promotion, advertisement, or medical advice.