- June 01, 2015
- Taiho Pharmaceutical Co., Ltd.
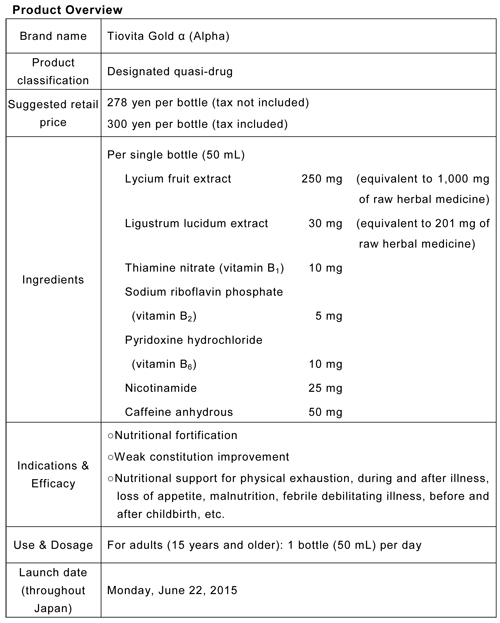
Support for modern people in the prime of life! Designated quasi-drug Tiovita Gold α (Alpha) mini-drink to be launched throughout Japan on June 22
Taiho Pharmaceutical Co., Ltd. (HQ: Tokyo, President and Representative Director: Masayuki Kobayashi) announced today that the first new designated quasi-drug in the Tiovita Gold series, Tiovita Gold α (Alpha) is to be pre-launched in some retail stores today, and launched throughout Japan on Monday, June 22. A TV commercial is to be broadcast throughout Japan from Wednesday, July 1.
The Tiovita Gold series consists of mini-drinks of 50mL or less with herbal medicine added to the original Tiovita Drink (designated quasi-drug) that is a household name in Japan. Since Tiovita Gold, the first product in the Tiovita Gold series, was introduced in 1982, the series has been a favorite of consumers for 33 years.
Taiho Pharmaceutical has developed Tiovita Gold α (Alpha) as a designated quasi-drug to deliver the same enjoyable smoothness and flavor of the Tiovita Gold (second-class OTC drug) to more consumers. The inclusion of herbal medicines to counter fatigue makes this product perfect for modern people in the prime of life who suffer from physical exhaustion.
Taiho Pharmaceutical anticipates that the Tiovita series will continue to make even greater contributions to healthy life in Japan.
Features of Tiovita Gold α (Alpha)
●The first in the Tiovita series to include Lycium fruit extract and Ligustrum lucidum extract, both herbal medicines with anti-fatigue action.
●Nearly the same flavor as Tiovita Gold (second-class OTC drug), which has a reputation for its smoothness and flavor.1
1 According to a 2014 user survey conducted by Taiho Pharmaceutical Co., Ltd.
Information in this news release was current as of the original release date.
Taiho Pharmaceutical's news releases are intended to provide information to the media. It may contain information about ethical drugs or products under development, however information contained in the news releases are not intended to constitute promotion, advertisement, or medical advice.