- September 01, 2015
- Taiho Pharmaceutical Co., Ltd.
The First Pre-Meal Drink in the Solmack Series "Solmack 5 Herbal Digestive Support" Nationwide Launch in Japan on Monday, September 7
Taiho Pharmaceutical Co., Ltd. (HQ: Tokyo, President and Representative Director: Masayuki Kobayashi) announced today that it will launch the plum-flavored and easy-to-drink herbal digestive support Solmack 5 (designated quasi-drug) nationwide in Japan on Monday, September 7. It is the first pre-meal drink in the Solmack Series, and a TV commercial will begin airing on Thursday, October 1.
-
-

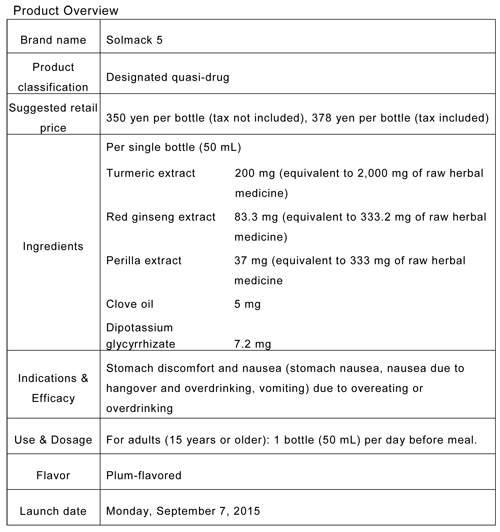
- Brand name: Solmack 5
-
Features of Solmack 5
Contains the maximum amount of turmeric allowed by the Designated Quasi-Drug Standards (equivalent in crude herb: 2,000 mg).
The first drink in the Solmack series specifically intended for pre-meal consumption.
Contains five types of herbs, including perilla and red ginseng, which are comparatively less bitter-tasting.
Plum flavor is easy to drink before a meal.
The Solmack Series is a line of natural herbal digestive drinks in bottles of 50 mL or less that are effective for hangovers and stomach nausea. Solmack products have remained popular for 36 years since the first product in this series, the natural herbal digestive medicine Solmack, was launched in 1979.
Solmack 5 features a refreshing and easy-to-drink plum flavor that was formulated specifically because the product is intended to be drunk before a meal. It contains herbs such as perilla and red ginseng, which are comparatively less bitter-tasting, to make it more appealing to consumers who don’t like the bitterness of other Solmack products.
Drinking Solmack 5 before a meal will help consumers with weak stomach to improve the stomach discomfort due to overeating or overdrinking.
Taiho Pharmaceutical anticipates that the Solmack series will continue to make even greater contributions to people’s health and happy lifestyles in Japan.
Information in this news release was current as of the original release date.
Taiho Pharmaceutical's news releases are intended to provide information to the media. It may contain information about ethical drugs or products under development, however information contained in the news releases are not intended to constitute promotion, advertisement, or medical advice.